Abstract
Introduction : We conducted a prospective study on T cell and NK cell subset composition of the graftand the transplant outcome with single dose of subcutaneous Plerixafor(Px) to GCSF based mobilisation in T-replete Haploidentical Peripheral blood Stem cell transplantation with post-transplantation cyclophosphamide (PTCy) in 25 donors (G+Px group). This was compared with 26 donors who received G-CSF alone for mobilisation (G group).
Material and Methods : With the aim to collect 10 x 106/kg CD34 cells in a single apheresis, donors with peripheral CD 34 count less than 50 cells /µl in the peripheral blood on day 4 of mobilization were administered Injection Plerixafor at the dose of 0.12mcg/kg at 12 midnight in addition to routine GCSF. This was followed by PBSC harvest starting 11 hours later at 11 A.M on the next day. The rest were continued G-CSF only as per protocol.
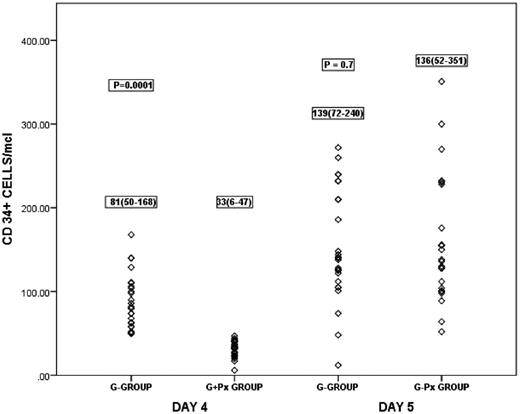
Results: CD 34 cell count[G+Px-136(range, 52-351)vs G-139 (range,72-240) cells/µl ] in the peripheral blood on day 5 as well as that in the graft [G+Px- 2.7(range 1.1-6.0) VsG-2.3(range 0.5-5.3) X106 /ml, p=0.05] comparable between the two groups, despite a significantly lower peripheral CD34 cell count on day 4 in the pleraxifor group (G+Px-33(range, 6-47) vs G-81(58-168) cells/µl, p=0.0001). The total nucleated cell count was higher in the G- group[ 3.4(range 1.7-5.0) vs 3.1(range 1.15-4.73) X108/ml, p=0.05 ]. Despite the lower trend of CD4+ T cells[ 2.3(range 0.3-6.8) Vs 2.7(range 0.53-6.8) X107/ml, p=0.09 ]in the G-CSF group, mobilisation of Tregs was similar(7.1% vs 6.9% in G+Px group,p=0.1). There were no differences noted in the absolute number of NK cells [G+Px-5.3(range 0.8-11.6) vs 5.7.(range 1.7-12.9) X106/ml, p=0.9 ], or the CD56+16+/16- subsets. The time to engraftment was similar in both groups. The incidences of acute and chronic GVHD, non-relapse mortality and relapse were also similar.
Conclusion: Addition of single dose pleraxifor to G-CSF mobilisation improves CD34 recovery and does not significantly alter the T and NK cell composition of the graft, including Tregs. No adverse impact was noted on engraftment, GVHD, NRM and relapse. Our study demonstrates that pleraxifor can be safely used as an adjunct to G-CSF to boost CD34 mobilisation without impacting conventional or regulatory T cells and NK cells in the graft. The preliminary data suggests no adverse impact on transplant outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal